Why Choose MITIGO™
The unique benefits of MITIGO allow patients to manage chronic pain and improve their quality of life.
20% of US adults experience chronic pain. MITIGO is changing the way their pain is managed.
Opioids are commonly prescribed for chronic pain. Yet oral opioids have been associated with abuse, addiction, and overdoses. Through its intrathecal delivery, MITIGO provides relief with a traceable method of opioid delivery, cutting down on the risks of overuse and abuse.
Other reasons to choose MITIGO:
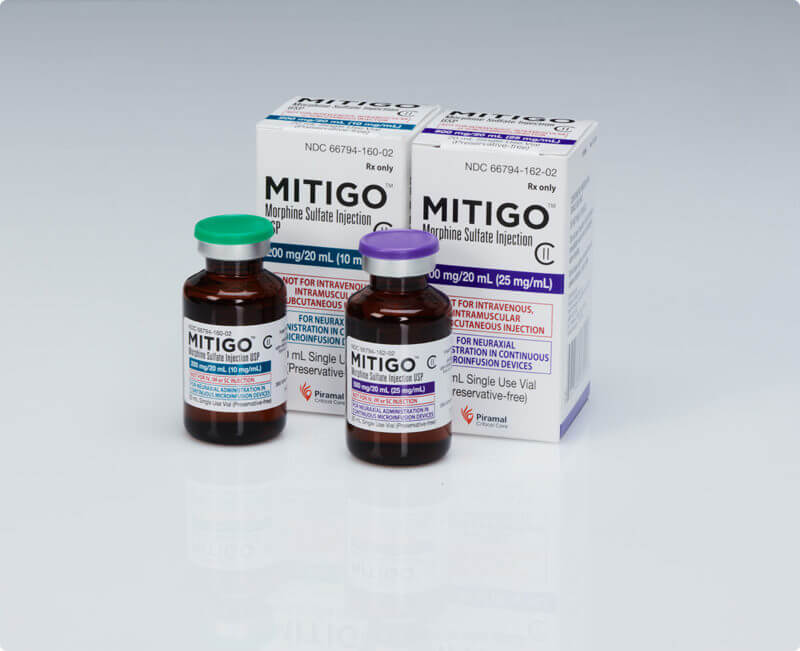
FDA-approved, non-compounded injectable morphine dosage
- MITIGO is the only FDA-approved morphine sulfate injection available in a vial.
- The non-compounded formula means that there’s no variation in potency. It’s safer for the patient because they know they are getting the same dosage each time.
- Non-compounded drugs are preferred for use in continuous microinfusion devices as they don’t clog the device.
- Off-label drug monotherapy or combination therapy, including using compounded morphine sulfate, is not recommended until FDA-approved drugs, like MITIGO, are tried and failed or contraindicated.10

Traceable Method of Opioid Dispensing and Consumption
- The administration of MITIGO provides a traceable and accountable method for dispensing and consumption of morphine sulfate. Individualized dosing is calculated by the clinician.
- This approach may help reduce the risk of deviating from an appropriate dosing regimen.
- The delivery method means reduced risks of opioid abuse, addiction, and overdose.
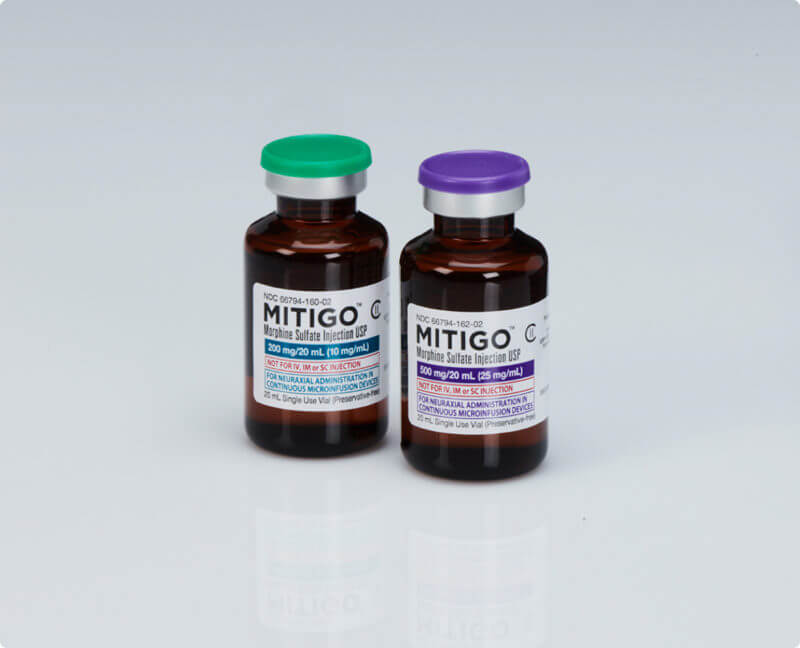
Morphine Sulfate Injection is safer, more convenient, easier-to-use, less wasteful than glass ampules
- MITIGO comes packaged in convenient, easy-to-use, and preservative-free amber vials. No need to fill syringes!
- With MITIGO, you don’t have to worry about breaking glass ampules or cutting yourself on glass fragments or chips for a morphine sulfate dosage
- Glass particulates and bacterial contamination have long been recognized as potential hazards associated with the cracking of ampules,9 and an estimated 62%-88% of sharps injuries can be prevented by using safer medical devices.5
- The longer shelf life (2 years) compared to compounded drugs (24-72 hours) mean less drug waste.

Part of the Piramal Critical Care family
- Access to Piramal Critical Care’s experienced and dedicated team, ready to bring you customized support to meet your specific needs.
- Participate in our educational programs, including peer-to-peer educational programs, roundtables, and on-site presentations and trainings.
- Provide feedback and insights so we can continually improve our line of intrathecal drugs and ensure they are easy-to-use and have optimal usability.
- As the world’s largest approved intrathecal product company, and only company to have both approved morphine and baclofen, we can be your dedicated intrathecal team.
Download MITIGO Brochure
Download the full brochure to learn more about how to manage your patients’ chronic pain.
*Education programs, trials, and materials including brochures, videos, and webinars, are only available for US-based companies.
Do You Have a Specific Question?
Important Risk Information
INDICATIONS AND USAGE
MITIGO™ (Morphine Sulfate Injection, USP – Preservative-free) is an opioid agonist, for use in continuous microinfusion devices and indicated only for intrathecal or epidural infusion in the management of intractable chronic pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
IMPORTANT RISK INFORMATION
WARNING: RISKS WITH NEURAXIAL ADMINISTRATION; LIFE-THREATENING RESPIRATORY DEPRESSION; RISK OF ADDICTION, ABUSE, AND MISUSE; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
See full prescribing information for complete boxed warning.
- Single-dose neuraxial administration may result in acute or delayed respiratory depression up to 24 hours. Because of the risk of severe adverse reactions when MITIGO is administered by the epidural or intrathecal route of administration, patients must be observed in a fully equipped and staffed environment for at least 24 hours after the initial dose.
- Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely, especially upon initiation or following a dose increase. Patients must be observed in a fully equipped and staffed environment for at least 24 hours after each test dose and, as indicated, for the first several days after surgery.
- MITIGO exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess patient’s risk before prescribing and monitor regularly for these behaviors and conditions.
- Prolonged use of MITIGO during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated. If prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available.
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation.
CONTRAINDICATIONS
- Significant respiratory depression
- Acute or severe bronchial asthma in an unmonitored setting in absence of resuscitative equipment
- Concurrent use of monoamine oxidase inhibitors (MAOIs) or use of MAOIs within the last 14 days
- Known or suspected gastrointestinal obstruction, including paralytic ileus
- Hypersensitivity or intolerance to morphine
Neuraxial administration of MITIGO is contraindicated in patients with:
- Infection at the injection microinfusion site
- Concomitant anticoagulant therapy
- Uncontrolled bleeding diathesis
- The presence of any other concomitant therapy or medical condition which would render epidural or intrathecal administration of medication especially hazardous.
WARNINGS AND PRECAUTIONS
- Risk of Inflammatory Masses: Monitor patients receiving continuous infusion of MITIGO via indwelling intrathecal catheter for new signs or symptoms of neurologic impairment.
- Risk of Tolerance and Myoclonic Activity: Monitor patients for unusual acceleration of neuraxial morphine, which may cause myoclonic-like spasm of lower extremities. Detoxification may be required.
- Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Monitor closely, particularly during initiation and titration.
- Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid.
- Severe Hypotension: Monitor during dosage initiation and titration. Avoid use of MITIGO in patients with circulatory shock.
- Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Monitor for sedation and respiratory depression. Avoid use of MITIGO in patients with impaired consciousness or coma.
ADVERSE REACTIONS
Most serious adverse reactions were respiratory depression, apnea, circulatory depression, respiratory arrest, shock, and cardiac arrest. Other common frequently observed adverse reactions include: sedation, lightheadedness, dizziness, nausea, vomiting, and constipation.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Hepatic and Renal Impairment: May affect the metabolism and excretion of MITIGO.
To report SUSPECTED ADVERSE REACTIONS, contact Piramal Critical Care, Inc. at 1-888-822-8431 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For additional Important Risk Information, including boxed warning, see enclosed Full Prescribing Information.
INDICATIONS AND USAGE
MITIGO™ (Morphine Sulfate Injection, USP – Preservative-free) is an opioid agonist, for use in continuous microinfusion devices and indicated only for intrathecal or epidural infusion in the management of intractable chronic pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
IMPORTANT RISK INFORMATION
WARNING: RISKS WITH NEURAXIAL ADMINISTRATION; LIFE-THREATENING RESPIRATORY DEPRESSION; RISK OF ADDICTION, ABUSE, AND MISUSE; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
See full prescribing information for complete boxed warning.
- Single-dose neuraxial administration may result in acute or delayed respiratory depression up to 24 hours. Because of the risk of severe adverse reactions when MITIGO is administered by the epidural or intrathecal route of administration, patients must be observed in a fully equipped and staffed environment for at least 24 hours after the initial dose.
- Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely, especially upon initiation or following a dose increase. Patients must be observed in a fully equipped and staffed environment for at least 24 hours after each test dose and, as indicated, for the first several days after surgery.
- MITIGO exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess patient’s risk before prescribing and monitor regularly for these behaviors and conditions.
- Prolonged use of MITIGO during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated. If prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available.
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation.
CONTRAINDICATIONS
- Significant respiratory depression
- Acute or severe bronchial asthma in an unmonitored setting in absence of resuscitative equipment
- Concurrent use of monoamine oxidase inhibitors (MAOIs) or use of MAOIs within the last 14 days
- Known or suspected gastrointestinal obstruction, including paralytic ileus
- Hypersensitivity or intolerance to morphine
Neuraxial administration of MITIGO is contraindicated in patients with:
- Infection at the injection microinfusion site
- Concomitant anticoagulant therapy
- Uncontrolled bleeding diathesis
- The presence of any other concomitant therapy or medical condition which would render epidural or intrathecal administration of medication especially hazardous.
WARNINGS AND PRECAUTIONS
- Risk of Inflammatory Masses: Monitor patients receiving continuous infusion of MITIGO via indwelling intrathecal catheter for new signs or symptoms of neurologic impairment.
- Risk of Tolerance and Myoclonic Activity: Monitor patients for unusual acceleration of neuraxial morphine, which may cause myoclonic-like spasm of lower extremities. Detoxification may be required.
- Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Monitor closely, particularly during initiation and titration.
- Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid.
- Severe Hypotension: Monitor during dosage initiation and titration. Avoid use of MITIGO in patients with circulatory shock.
- Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Monitor for sedation and respiratory depression. Avoid use of MITIGO in patients with impaired consciousness or coma.
ADVERSE REACTIONS
Most serious adverse reactions were respiratory depression, apnea, circulatory depression, respiratory arrest, shock, and cardiac arrest. Other common frequently observed adverse reactions include: sedation, lightheadedness, dizziness, nausea, vomiting, and constipation.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Hepatic and Renal Impairment: May affect the metabolism and excretion of MITIGO.
To report SUSPECTED ADVERSE REACTIONS, contact Piramal Critical Care, Inc. at 1-888-822-8431 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For additional Important Risk Information, including boxed warning, see enclosed Full Prescribing Information.